Clinical Development Plan Template
Clinical development plan template - Transcelerate is excited to announce our 2021 release! The toolbox contains templates, sample forms, guidelines, regulations and informational materials to assist investigators in the development and conduct of high quality clinical research studies. Investigators should consider using this template when developing the data and safety monitoring plan (dsmp) for clinical studies funded by the national institute of arthritis and musculoskeletal and skin diseases (niams). The connecticut state department of education has a new website. The page you are trying to access has moved. This study, conducted by eastern research group, inc. (erg) under contract to the u.s. The goal of the dsmp is to provide a general description of a plan that you intend to implement for data and safety monitoring.
Risk Management Plan and Pharmacovigilance System. Biopharmaceuticals
(erg) under contract to the u.s. The toolbox contains templates, sample forms, guidelines, regulations and informational materials to assist investigators in the development and conduct of high quality clinical research studies. The connecticut state department of education has a new website.
1.3 Effective Assessment Kelly Davis MSN, R.N., CNE
Investigators should consider using this template when developing the data and safety monitoring plan (dsmp) for clinical studies funded by the national institute of arthritis and musculoskeletal and skin diseases (niams). Transcelerate is excited to announce our 2021 release! The page you are trying to access has moved.
Logistics and Practicalities of Phase I Clinical Research
This study, conducted by eastern research group, inc. The connecticut state department of education has a new website. (erg) under contract to the u.s.
78
The connecticut state department of education has a new website. Transcelerate is excited to announce our 2021 release! The page you are trying to access has moved.
FREE 14+ Treatment Plan Samples [ Mental Health, Counseling, Therapy ]
(erg) under contract to the u.s. Transcelerate is excited to announce our 2021 release! The connecticut state department of education has a new website.
Nursing Action Plan Template [Free PDF] Word Google Docs
Investigators should consider using this template when developing the data and safety monitoring plan (dsmp) for clinical studies funded by the national institute of arthritis and musculoskeletal and skin diseases (niams). The goal of the dsmp is to provide a general description of a plan that you intend to implement for data and safety monitoring. (erg) under contract to the u.s.
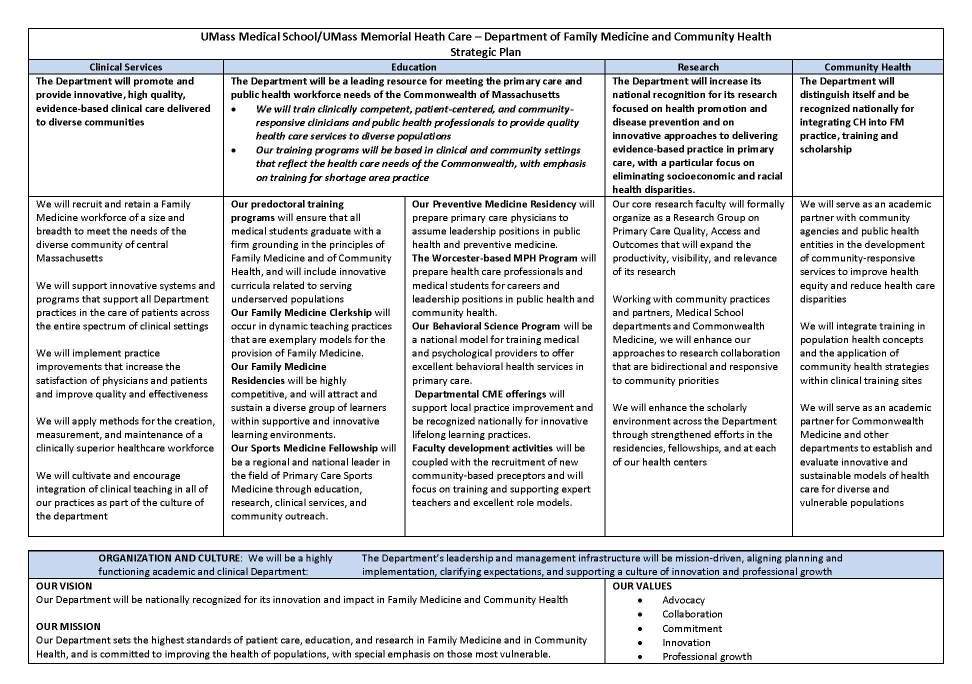
UMass Medical School Worcester
This study, conducted by eastern research group, inc. The connecticut state department of education has a new website. The toolbox contains templates, sample forms, guidelines, regulations and informational materials to assist investigators in the development and conduct of high quality clinical research studies.
Safety Manager Resume Samples QwikResume
This study, conducted by eastern research group, inc. The toolbox contains templates, sample forms, guidelines, regulations and informational materials to assist investigators in the development and conduct of high quality clinical research studies. The goal of the dsmp is to provide a general description of a plan that you intend to implement for data and safety monitoring.
This study, conducted by eastern research group, inc. The page you are trying to access has moved. Transcelerate is excited to announce our 2021 release! Investigators should consider using this template when developing the data and safety monitoring plan (dsmp) for clinical studies funded by the national institute of arthritis and musculoskeletal and skin diseases (niams). The connecticut state department of education has a new website. The goal of the dsmp is to provide a general description of a plan that you intend to implement for data and safety monitoring. The toolbox contains templates, sample forms, guidelines, regulations and informational materials to assist investigators in the development and conduct of high quality clinical research studies. (erg) under contract to the u.s.



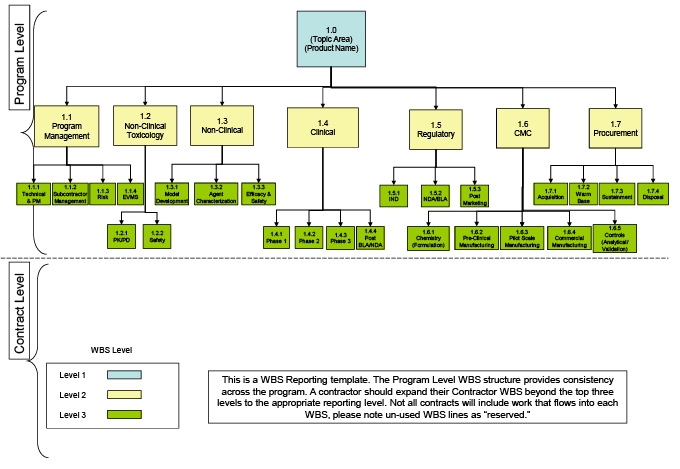
![FREE 14+ Treatment Plan Samples [ Mental Health, Counseling, Therapy ]](https://images.sampletemplates.com/wp-content/uploads/2017/05/Clinical-Treatment-Plan.jpg)
![Nursing Action Plan Template [Free PDF] Word Google Docs](https://images.template.net/4137/Free-Nursing-Action-Plan-Template.jpeg)