Installation Qualification Template
Installation qualification template - Built and installed in compliance with their design specifications (this constitutes installation qualification or ‘iq’) and that they operate in accordance with their design specifications (this constitutes operational qualification or oq). An installation qualification template is used to complete the process validation protocol by properly documenting that the equipment/system is correctly installed, supplied as specified, and integrated in the manufacturing facility calibration and maintenance systems. Concrete, paint, and stucco washout and waste disposal; Performance qualification is a part of equipment validation process and there are a number of reasons why pharmaceutical plants should perform it. And dewatering operations) and indicate for each activity the associated pollutants or pollutant constituents (e.g., sediment, fertilizers, pesticides, paints, caulks, sealants, fluorescent light. Operational qualification is usually performed before the system is released for use. Service life cycle management is a critical element when purchasing your equipment. Overview of the c&g 2357 nvq level 3 electrical installation testing structure. The operational qualification test requirements are defined in the functional requirements specification. $19.99 resume coach review credit.
Solid waste storage and disposal; Transcelerate is excited to announce our 2021 release! Proper life cycle management starts with the proper installation of the equipment and basic user training, which is followed by routine preventative maintenance visits. First of all, at the end of the day, if the companies intend to deliver quality products, then it becomes an ethical obligation for them to put their equipment through the performance qualification. 3.4 the portfolio.3.4.1 guidance on collation;
Creation of Installation Qualification Protocols
Get pro membership for only $9.99. The operational qualification test requirements are defined in the functional requirements specification. Performance qualification is a part of equipment validation process and there are a number of reasons why pharmaceutical plants should perform it.
Senior Estimator Resume Samples QwikResume
Operational qualification is usually performed before the system is released for use. Performance qualification is a part of equipment validation process and there are a number of reasons why pharmaceutical plants should perform it. The operational qualification protocol is a collection of test cases used to verify the proper functioning of a system.
Writing Compliant IQ/OQ/PQ Protocols M A N O X B L O G
Built and installed in compliance with their design specifications (this constitutes installation qualification or ‘iq’) and that they operate in accordance with their design specifications (this constitutes operational qualification or oq). 3.4 the portfolio.3.4.1 guidance on collation; Solid waste storage and disposal;
ISO 13485 Document Control Procedure Bundle
Solid waste storage and disposal; Transcelerate is excited to announce our 2021 release! Get pro membership for only $9.99.
Nvq Level 3 Certificate certificates templates free
Get pro membership for only $9.99. $19.99 resume coach review credit. And dewatering operations) and indicate for each activity the associated pollutants or pollutant constituents (e.g., sediment, fertilizers, pesticides, paints, caulks, sealants, fluorescent light.
IOPQ Laminar Air Flow Validation Template Sample by Pharmi Med Ltd
An installation qualification template is used to complete the process validation protocol by properly documenting that the equipment/system is correctly installed, supplied as specified, and integrated in the manufacturing facility calibration and maintenance systems. First of all, at the end of the day, if the companies intend to deliver quality products, then it becomes an ethical obligation for them to put their equipment through the performance qualification. Service life cycle management is a critical element when purchasing your equipment.
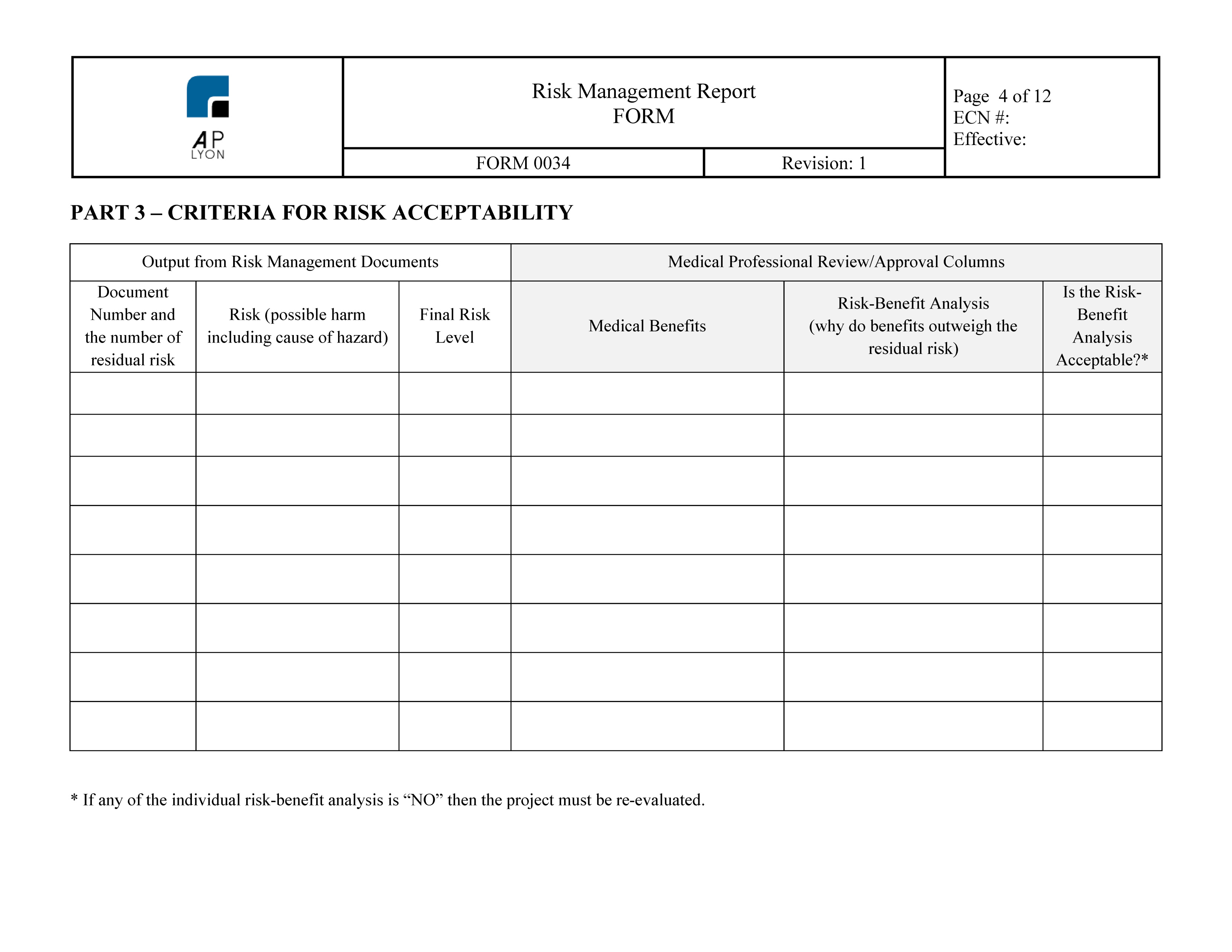
ISO 14971 Risk Management Forms
Proper life cycle management starts with the proper installation of the equipment and basic user training, which is followed by routine preventative maintenance visits. Get pro membership for only $9.99. A specific process will consistently produce a product meeting its predetermined specifications and
ISO 13485 Document Control Procedure Bundle
3.4 the portfolio.3.4.1 guidance on collation; Transcelerate is excited to announce our 2021 release! It does not matter what industry you are in, pharma, biopharma, university, or food & beverage;
3.4 the portfolio.3.4.1 guidance on collation; Operational qualification is usually performed before the system is released for use. Service life cycle management is a critical element when purchasing your equipment. The operational qualification test requirements are defined in the functional requirements specification. First of all, at the end of the day, if the companies intend to deliver quality products, then it becomes an ethical obligation for them to put their equipment through the performance qualification. And dewatering operations) and indicate for each activity the associated pollutants or pollutant constituents (e.g., sediment, fertilizers, pesticides, paints, caulks, sealants, fluorescent light. Also includes reference to the final am2 assessment. Concrete, paint, and stucco washout and waste disposal; The operational qualification protocol is a collection of test cases used to verify the proper functioning of a system. Performance qualification is a part of equipment validation process and there are a number of reasons why pharmaceutical plants should perform it.
An installation qualification template is used to complete the process validation protocol by properly documenting that the equipment/system is correctly installed, supplied as specified, and integrated in the manufacturing facility calibration and maintenance systems. Built and installed in compliance with their design specifications (this constitutes installation qualification or ‘iq’) and that they operate in accordance with their design specifications (this constitutes operational qualification or oq). Proper life cycle management starts with the proper installation of the equipment and basic user training, which is followed by routine preventative maintenance visits. Solid waste storage and disposal; Get pro membership for only $9.99. It does not matter what industry you are in, pharma, biopharma, university, or food & beverage; A specific process will consistently produce a product meeting its predetermined specifications and Overview of the c&g 2357 nvq level 3 electrical installation testing structure. $19.99 resume coach review credit. Transcelerate is excited to announce our 2021 release!