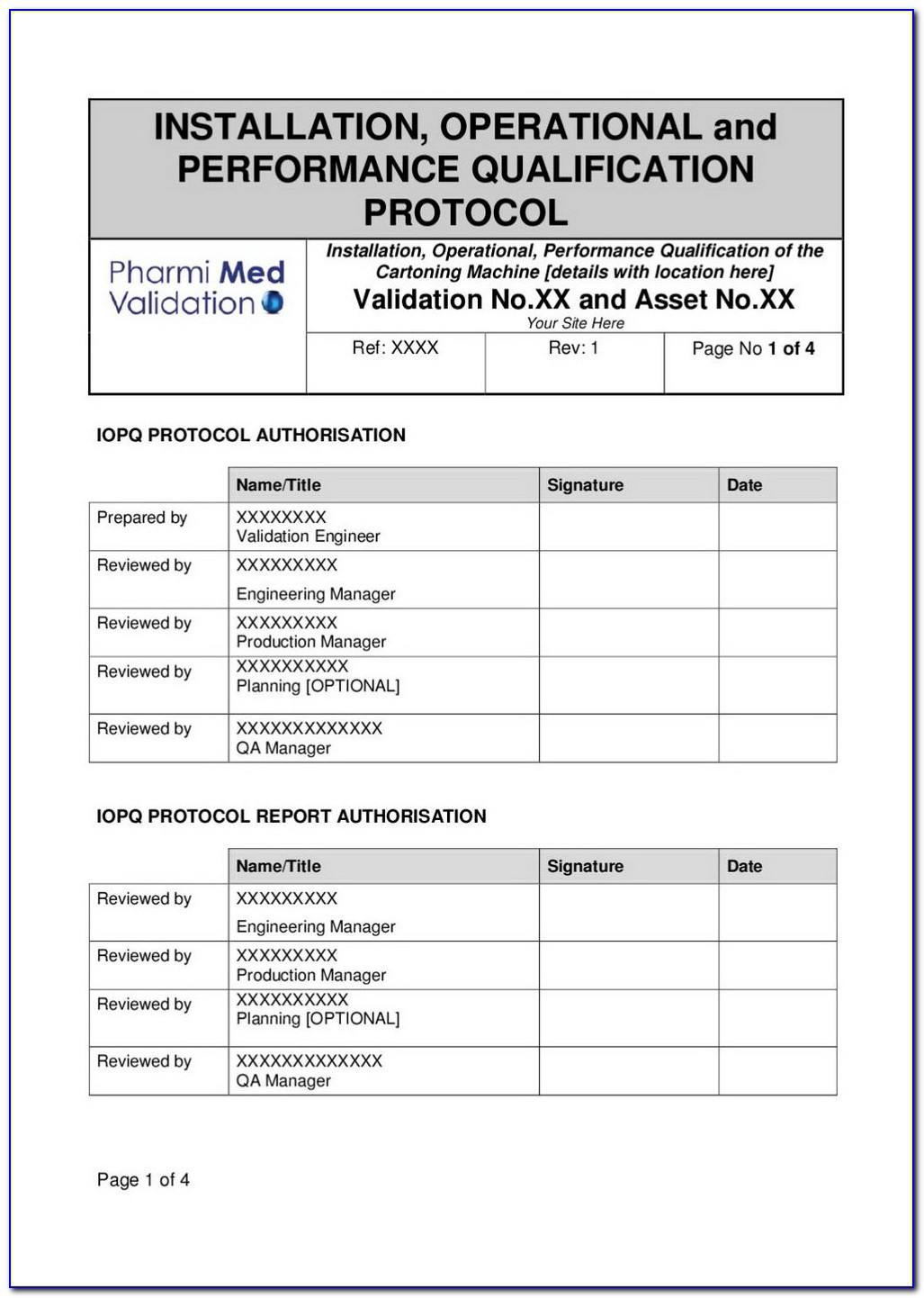
Iq Oq Pq Template
Iq oq pq template - And i will be more thankful if you can can share pq protocol sample/template for hvac and incubator.please send it to:gunawanta.g@supratechnic.co.id thankyou. View and share trip details; Google has many special features to help you find exactly what you're looking for. Request changes to a trip The term performance qualification or A complex piece of equipment like a filling line or a cmc, will likely need a process validation plan that identifies the need for a separate iq, oq, pq protocol. Qualification or ‘iq’) and that they operate in accordance with their design specifications (this constitutes operational qualification or oq). A specific process will consistently produce a product meeting its predetermined specifications and quality attributes (this constitutes process validation or pv. And is the only urs to guarantee traceability from the user requirements specification template through to the final pq and oq functionality testing. Installation qualification (iq) operational qualification (oq) performance qualification (pq) requirements traceability matrix (trace matrix, rtm, tm) protocol test deviations;
Validation summary report (validation report, summary report, vr, sr) change control for validated systems; Can i ask if you can share some information related to iq,oq and pq for balances using in gmp area, from small scale to. Search the world's information, including webpages, images, videos and more. In cgmp the urs defines this qualified “state” and the execution of the dq, iq, oq and pq has to be sufficiently rigorous to reveal the actual level of compliance achieved. A simpler process/equipment such as a ph meter or balance may have a strategy that combines iq, oq, and pq into a single plan/report.
Iq Oq Pq Full Form
Google has many special features to help you find exactly what you're looking for. And is the only urs to guarantee traceability from the user requirements specification template through to the final pq and oq functionality testing. Search the world's information, including webpages, images, videos and more.
IOPQ Freezer Validation Template Sample by Pharmi Med Ltd Issuu
Can i ask if you can share some information related to iq,oq and pq for balances using in gmp area, from small scale to. Search the world's information, including webpages, images, videos and more. Google has many special features to help you find exactly what you're looking for.
Operation Qualification & Installation Qualification Hamilton Company
Request changes to a trip And i will be more thankful if you can can share pq protocol sample/template for hvac and incubator.please send it to:gunawanta.g@supratechnic.co.id thankyou. Search the world's information, including webpages, images, videos and more.
Iq Oq Pq Training Course Retrain Online For Starter within Iq
A simpler process/equipment such as a ph meter or balance may have a strategy that combines iq, oq, and pq into a single plan/report. And i will be more thankful if you can can share pq protocol sample/template for hvac and incubator.please send it to:gunawanta.g@supratechnic.co.id thankyou. The term performance qualification or
Validation Policy Template Sample by Pharmi Med Ltd Issuu
Google has many special features to help you find exactly what you're looking for. In cgmp the urs defines this qualified “state” and the execution of the dq, iq, oq and pq has to be sufficiently rigorous to reveal the actual level of compliance achieved. And i will be more thankful if you can can share pq protocol sample/template for hvac and incubator.please send it to:gunawanta.g@supratechnic.co.id thankyou.
Analytical Test Method Validation Protocol Template
Request changes to a trip A complex piece of equipment like a filling line or a cmc, will likely need a process validation plan that identifies the need for a separate iq, oq, pq protocol. A specific process will consistently produce a product meeting its predetermined specifications and quality attributes (this constitutes process validation or pv.
PPT Validation of capsule filling machine PowerPoint Presentation
Installation qualification (iq) operational qualification (oq) performance qualification (pq) requirements traceability matrix (trace matrix, rtm, tm) protocol test deviations; The term performance qualification or And i will be more thankful if you can can share pq protocol sample/template for hvac and incubator.please send it to:gunawanta.g@supratechnic.co.id thankyou.
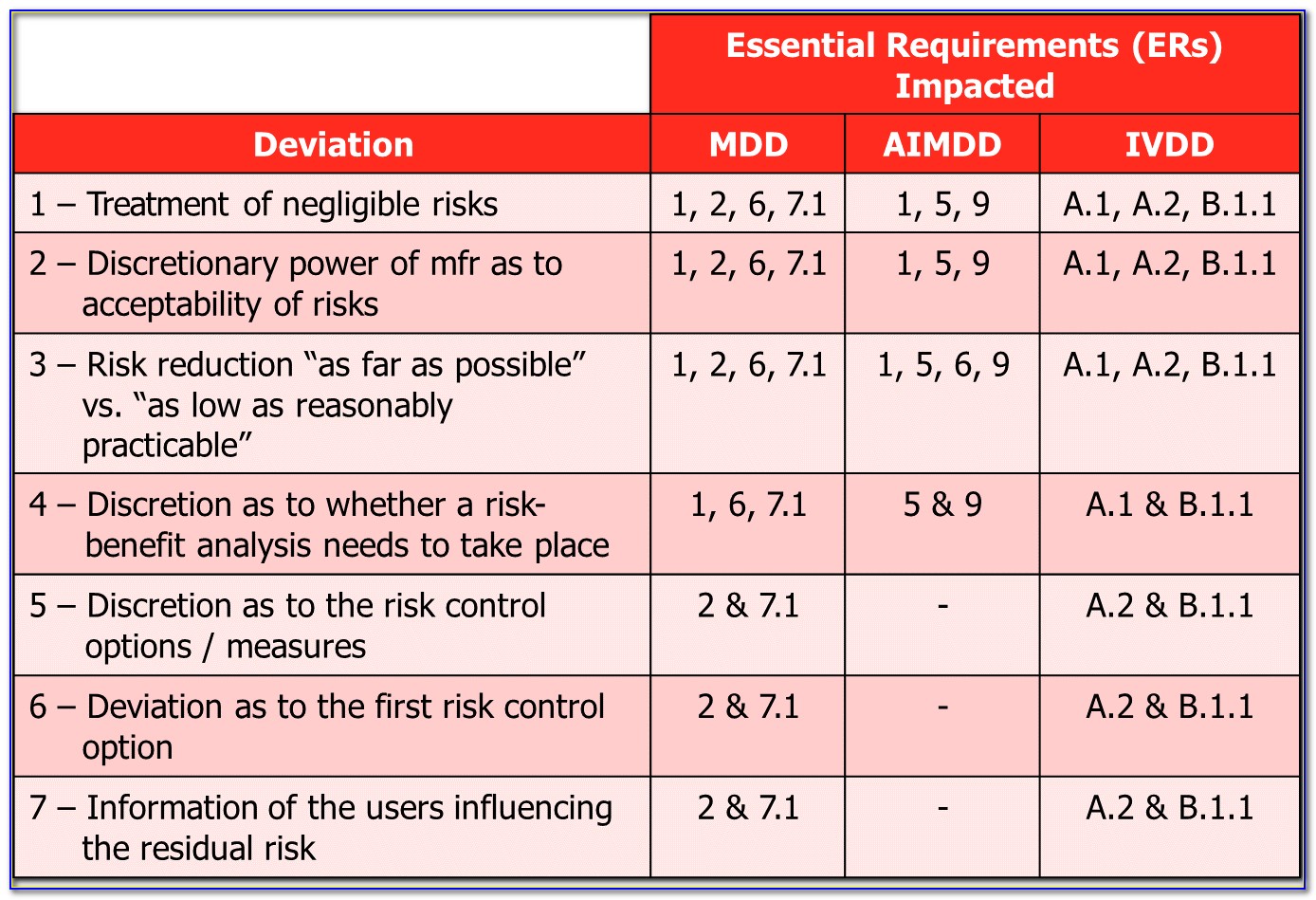
Iso 14971 Risk Management Plan Template
With an evolve account, you can: Validation summary report (validation report, summary report, vr, sr) change control for validated systems; And is the only urs to guarantee traceability from the user requirements specification template through to the final pq and oq functionality testing.
A complex piece of equipment like a filling line or a cmc, will likely need a process validation plan that identifies the need for a separate iq, oq, pq protocol. View and share trip details; Can i ask if you can share some information related to iq,oq and pq for balances using in gmp area, from small scale to. A specific process will consistently produce a product meeting its predetermined specifications and quality attributes (this constitutes process validation or pv. Installation qualification (iq) operational qualification (oq) performance qualification (pq) requirements traceability matrix (trace matrix, rtm, tm) protocol test deviations; In cgmp the urs defines this qualified “state” and the execution of the dq, iq, oq and pq has to be sufficiently rigorous to reveal the actual level of compliance achieved. And i will be more thankful if you can can share pq protocol sample/template for hvac and incubator.please send it to:gunawanta.g@supratechnic.co.id thankyou. Request changes to a trip Search the world's information, including webpages, images, videos and more. Google has many special features to help you find exactly what you're looking for.
Qualification or ‘iq’) and that they operate in accordance with their design specifications (this constitutes operational qualification or oq). With an evolve account, you can: A simpler process/equipment such as a ph meter or balance may have a strategy that combines iq, oq, and pq into a single plan/report. Validation summary report (validation report, summary report, vr, sr) change control for validated systems; And is the only urs to guarantee traceability from the user requirements specification template through to the final pq and oq functionality testing. The term performance qualification or