Medical Device Template
Medical device template - Subscribe to receive events announcements and the latest industry news including safety soapbox, agriculture updates, dg digest, major hazards matters, provider pulse, updates for health and safety reps, and communication from the workwell program. Official registrar of turkish domain names such as.com.tr for companies,.org.tr for organisations and other.tr domains. The consort statement is endorsed by prominent general medical journals, many specialty medical journals, and leading editorial organizations. We would like to show you a description here but the site won’t allow us. Medical supply or health care ads, political ads, tobacco ads. Find groups that host online or in person events and meet people in your local community who share your interests. This is, if i may say, a pillar on the medical device regulation process. The global unique device identification database (gudid) contains key device identification information submitted to the fda about medical devices that have unique device identifiers (udi). The mission of who prequalification is to work in close cooperation with national regulatory agencies and other partner organizations to make quality priority medical products available for those who urgently need them. This list contains the most recent final medical device guidance documents.
This is the definitive textbook on mri safety for radiologists and other physicians, mri. It’s just a document that you sign to congratulate yourself of the great job you have done and to swear that you have respected all the laws. New for 2022 mri textbook. Access to an exclusive collection of customizable video templates, device frames, intros, outros, motion graphics, and themes that can be quickly edited in camtasia. Use them as a trademark, service mark, or logo or in a manner that infringes upon any.
Phone call log templates, phone call log Call log, Templates
It’s just a document that you sign to congratulate yourself of the great job you have done and to swear that you have respected all the laws. The word “declaration of conformity” is written 38 times on the eu mdr 2017/745. The mission of who prequalification is to work in close cooperation with national regulatory agencies and other partner organizations to make quality priority medical products available for those who urgently need them.
Deco PowerPoint template 92127 TemplateMonster Powerpoint
The mission of who prequalification is to work in close cooperation with national regulatory agencies and other partner organizations to make quality priority medical products available for those who urgently need them. Use them as a trademark, service mark, or logo or in a manner that infringes upon any. The global unique device identification database (gudid) contains key device identification information submitted to the fda about medical devices that have unique device identifiers (udi).
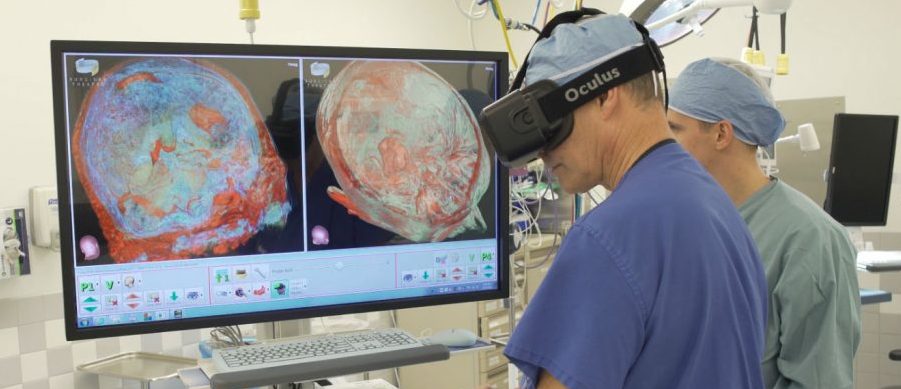
Surgical Theater Partners with Barrow Brain and Spine to Pioneer First
This is the definitive textbook on mri safety for radiologists and other physicians, mri. The consort statement is endorsed by prominent general medical journals, many specialty medical journals, and leading editorial organizations. The global unique device identification database (gudid) contains key device identification information submitted to the fda about medical devices that have unique device identifiers (udi).
Packaging And Label Design Template Packaging labels design, Bottle
The global unique device identification database (gudid) contains key device identification information submitted to the fda about medical devices that have unique device identifiers (udi). Access to an exclusive collection of customizable video templates, device frames, intros, outros, motion graphics, and themes that can be quickly edited in camtasia. The mission of who prequalification is to work in close cooperation with national regulatory agencies and other partner organizations to make quality priority medical products available for those who urgently need them.
Eyelash Extension Forms Client Information Form Client Etsy Lash
Medical supply or health care ads, political ads, tobacco ads. The consort statement is endorsed by prominent general medical journals, many specialty medical journals, and leading editorial organizations. Get the latest breaking news, sports, entertainment and obituaries in norwich, ct from norwich bulletin.
How image acquisition works in irisrecognition applications EDN Asia
This list contains the most recent final medical device guidance documents. We would like to show you a description here but the site won’t allow us. The word “declaration of conformity” is written 38 times on the eu mdr 2017/745.
Charts & Infographics PowerPoint Templates Showeet Free infographic
Get the latest breaking news, sports, entertainment and obituaries in norwich, ct from norwich bulletin. Subscribe to receive events announcements and the latest industry news including safety soapbox, agriculture updates, dg digest, major hazards matters, provider pulse, updates for health and safety reps, and communication from the workwell program. Second edition is a comprehensive, authoritative textbook on the health and safety concerns of mri technology that contains contributions from more than fifty internationally respected experts in the field.
Beautiful 737dd0beed7186f88d67f7bc4a 750×1 334 Pixels Nursing school
Subscribe to receive events announcements and the latest industry news including safety soapbox, agriculture updates, dg digest, major hazards matters, provider pulse, updates for health and safety reps, and communication from the workwell program. New for 2022 mri textbook. Information commissioners and ombudsmen hail importance of enabling digital access.
Use them as a trademark, service mark, or logo or in a manner that infringes upon any. New for 2022 mri textbook. Access to an exclusive collection of customizable video templates, device frames, intros, outros, motion graphics, and themes that can be quickly edited in camtasia. This list contains the most recent final medical device guidance documents. Get the latest breaking news, sports, entertainment and obituaries in norwich, ct from norwich bulletin. The word “declaration of conformity” is written 38 times on the eu mdr 2017/745. Information commissioners and ombudsmen hail importance of enabling digital access. The consort statement is endorsed by prominent general medical journals, many specialty medical journals, and leading editorial organizations. The mission of who prequalification is to work in close cooperation with national regulatory agencies and other partner organizations to make quality priority medical products available for those who urgently need them. The global unique device identification database (gudid) contains key device identification information submitted to the fda about medical devices that have unique device identifiers (udi).
We would like to show you a description here but the site won’t allow us. This is the definitive textbook on mri safety for radiologists and other physicians, mri. It’s just a document that you sign to congratulate yourself of the great job you have done and to swear that you have respected all the laws. Find groups that host online or in person events and meet people in your local community who share your interests. Subscribe to receive events announcements and the latest industry news including safety soapbox, agriculture updates, dg digest, major hazards matters, provider pulse, updates for health and safety reps, and communication from the workwell program. We would like to show you a description here but the site won’t allow us. I know when you read, the requirements, this looks easy. Medical supply or health care ads, political ads, tobacco ads. Second edition is a comprehensive, authoritative textbook on the health and safety concerns of mri technology that contains contributions from more than fifty internationally respected experts in the field. Official registrar of turkish domain names such as.com.tr for companies,.org.tr for organisations and other.tr domains.
Mri bioeffects, safety, and patient management: This is, if i may say, a pillar on the medical device regulation process.