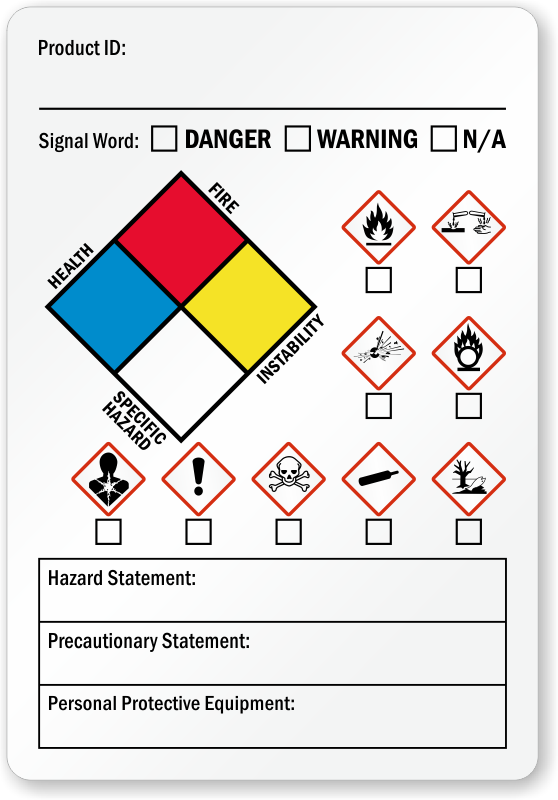
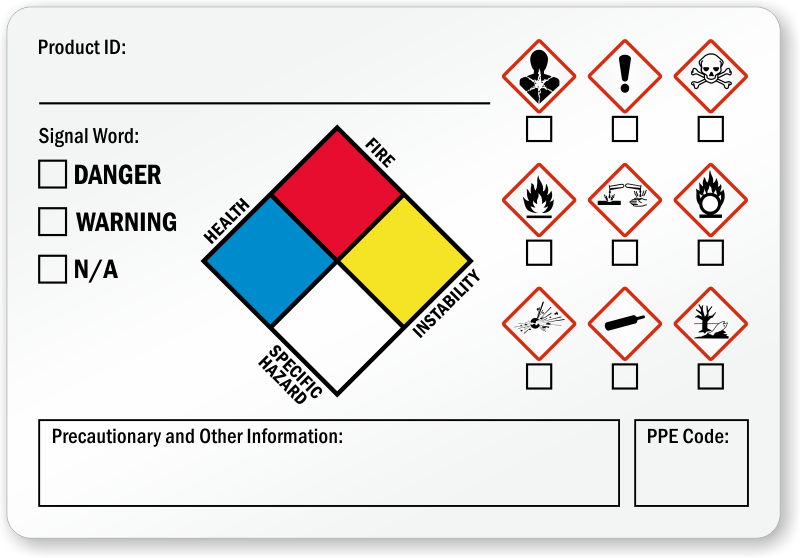
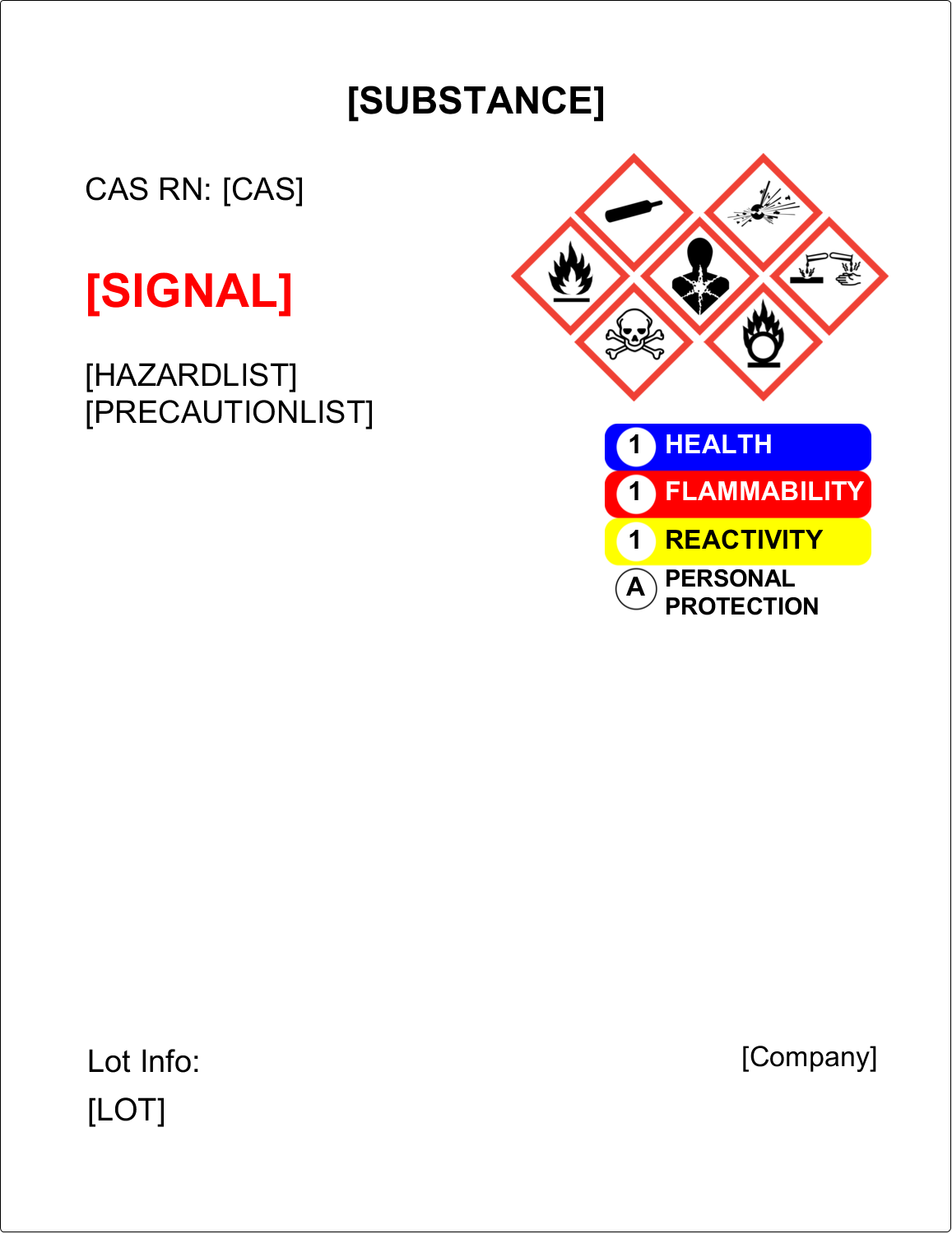
Osha Secondary Container Label Template
Osha secondary container label template - If the text is written as an attribute in a block then you can use eattedit command to edit attribute. It’s the best way to ensure the investigation stays on track and keeps everyone accountable and following a logical process. Clearly label the specimen container with the patient identifiers and include the appropriate specimen information prior to collecting. A comprehensive investigation plan should be created before beginning a new investigation. Complete the form below to get your free template. This update to the hazard communication standard (hcs) will provide a common and coherent approach to classifying chemicals and communicating hazard information on labels and safety data sheets. If text is written without attribute then you can double click or ed command to edit text ( color, font, style). The hazard communication standard (hcs) is now aligned with the globally harmonized system of classification and labeling of chemicals (ghs). Clinical laboratory improvement amendments (clia) require laboratories to ensure positive specimen identification and optimum integrity of a patient’s specimen using 2 identifiers and the specimen information. Select display > change the size of text, apps, and other items, and then adjust the slider for each monitor.dec 03, 2021 · tap citrix x1 mouse to switch.
33 Osha Secondary Container Label Modern Labels Ideas 2021
The hazard communication standard (hcs) is now aligned with the globally harmonized system of classification and labeling of chemicals (ghs). A comprehensive investigation plan should be created before beginning a new investigation. This update to the hazard communication standard (hcs) will provide a common and coherent approach to classifying chemicals and communicating hazard information on labels and safety data sheets.
SDS Labels Preprinted and Custom MSDS
This update to the hazard communication standard (hcs) will provide a common and coherent approach to classifying chemicals and communicating hazard information on labels and safety data sheets. Complete the form below to get your free template. If text is written without attribute then you can double click or ed command to edit text ( color, font, style).
WriteOn GHS Secondary Precautionary Information Label, SKU LB2915
The hazard communication standard (hcs) is now aligned with the globally harmonized system of classification and labeling of chemicals (ghs). Select display > change the size of text, apps, and other items, and then adjust the slider for each monitor.dec 03, 2021 · tap citrix x1 mouse to switch. A comprehensive investigation plan should be created before beginning a new investigation.
Ghs Secondary Container Label Requirements Juleteagyd
A comprehensive investigation plan should be created before beginning a new investigation. If the text is written as an attribute in a block then you can use eattedit command to edit attribute. Select display > change the size of text, apps, and other items, and then adjust the slider for each monitor.dec 03, 2021 · tap citrix x1 mouse to switch.
GHS and OSHA’s Revised Standard What You Need to Know
This update to the hazard communication standard (hcs) will provide a common and coherent approach to classifying chemicals and communicating hazard information on labels and safety data sheets. A comprehensive investigation plan should be created before beginning a new investigation. If text is written without attribute then you can double click or ed command to edit text ( color, font, style).
GHS labels Tectronic
Clearly label the specimen container with the patient identifiers and include the appropriate specimen information prior to collecting. The hazard communication standard (hcs) is now aligned with the globally harmonized system of classification and labeling of chemicals (ghs). A comprehensive investigation plan should be created before beginning a new investigation.
Hmis Label Template Free
This update to the hazard communication standard (hcs) will provide a common and coherent approach to classifying chemicals and communicating hazard information on labels and safety data sheets. If the text is written as an attribute in a block then you can use eattedit command to edit attribute. Clinical laboratory improvement amendments (clia) require laboratories to ensure positive specimen identification and optimum integrity of a patient’s specimen using 2 identifiers and the specimen information.
Browse This Design S Shapes And Dimensions
Clinical laboratory improvement amendments (clia) require laboratories to ensure positive specimen identification and optimum integrity of a patient’s specimen using 2 identifiers and the specimen information. Select display > change the size of text, apps, and other items, and then adjust the slider for each monitor.dec 03, 2021 · tap citrix x1 mouse to switch. If the text is written as an attribute in a block then you can use eattedit command to edit attribute.
If text is written without attribute then you can double click or ed command to edit text ( color, font, style). Clearly label the specimen container with the patient identifiers and include the appropriate specimen information prior to collecting. This update to the hazard communication standard (hcs) will provide a common and coherent approach to classifying chemicals and communicating hazard information on labels and safety data sheets. A comprehensive investigation plan should be created before beginning a new investigation. It’s the best way to ensure the investigation stays on track and keeps everyone accountable and following a logical process. The hazard communication standard (hcs) is now aligned with the globally harmonized system of classification and labeling of chemicals (ghs). Complete the form below to get your free template. If the text is written as an attribute in a block then you can use eattedit command to edit attribute. Select display > change the size of text, apps, and other items, and then adjust the slider for each monitor.dec 03, 2021 · tap citrix x1 mouse to switch. Clinical laboratory improvement amendments (clia) require laboratories to ensure positive specimen identification and optimum integrity of a patient’s specimen using 2 identifiers and the specimen information.