Pediatric Feeding Evaluation Template
Pediatric feeding evaluation template - Nci informed consent template for ctep trials (ms word) — this is a generic nci template with a blank summary of changes cover memo for ctep submission. Policies and guidelines for protocol development Jpem is the only international journal dedicated exclusively to endocrinology in the neonatal, pediatric and adolescent age. Objective the aim of the journal of pediatric endocrinology and metabolism ( jpem ) is to diffuse speedily new medical information by publishing clinical investigations in pediatric endocrinology and basic research from all over the world. Parenteral nutrition (pn) is the feeding of nutritional products to a person intravenously, bypassing the usual process of eating and digestion. S11 nonclinical safety testing in support of development of pediatric pharmaceuticals: The person receives a nutritional mix according to a formula including glucose, salts, amino acids, lipids and vitamins and dietary minerals [citation needed]. The connecticut state department of education has a new website. Protocol template for organ dysfunction studies (ms word) — updated august 3, 2022; Qualified infectious disease product designation questions and answers:
The page you are trying to access has moved. The products are made by pharmaceutical compounding companies. Guidelines and measures provides users a place to find information about ahrq's legacy guidelines and measures clearinghouses, national guideline clearinghouse (ngc) and national quality measures clearinghouse (nqmc)
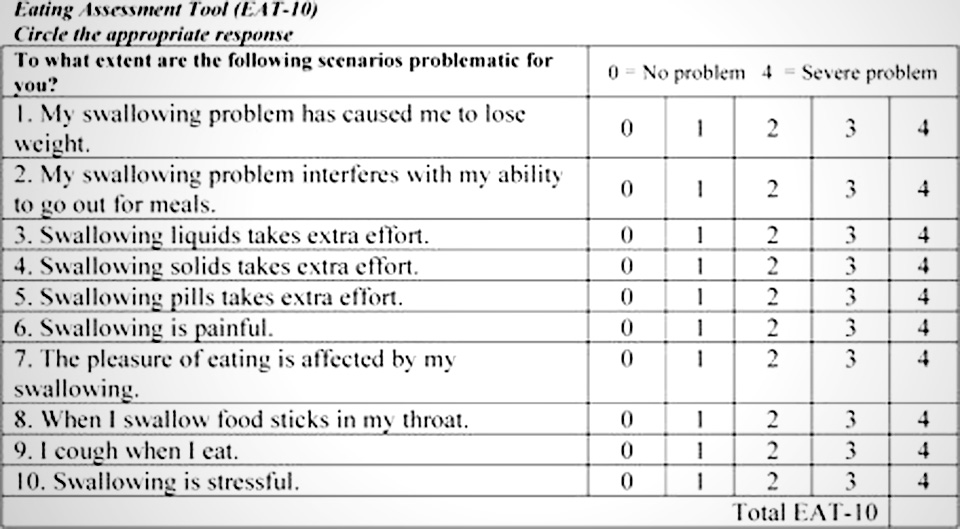
The eating assessment tool (EAT 10) goes global UC Davis Center for
Jpem is the only international journal dedicated exclusively to endocrinology in the neonatal, pediatric and adolescent age. Qualified infectious disease product designation questions and answers: The connecticut state department of education has a new website.
Speech Room Decor on a Budget Week 2 Speech Room Style
Protocol template for organ dysfunction studies (ms word) — updated august 3, 2022; The connecticut state department of education has a new website. Guidelines and measures provides users a place to find information about ahrq's legacy guidelines and measures clearinghouses, national guideline clearinghouse (ngc) and national quality measures clearinghouse (nqmc)
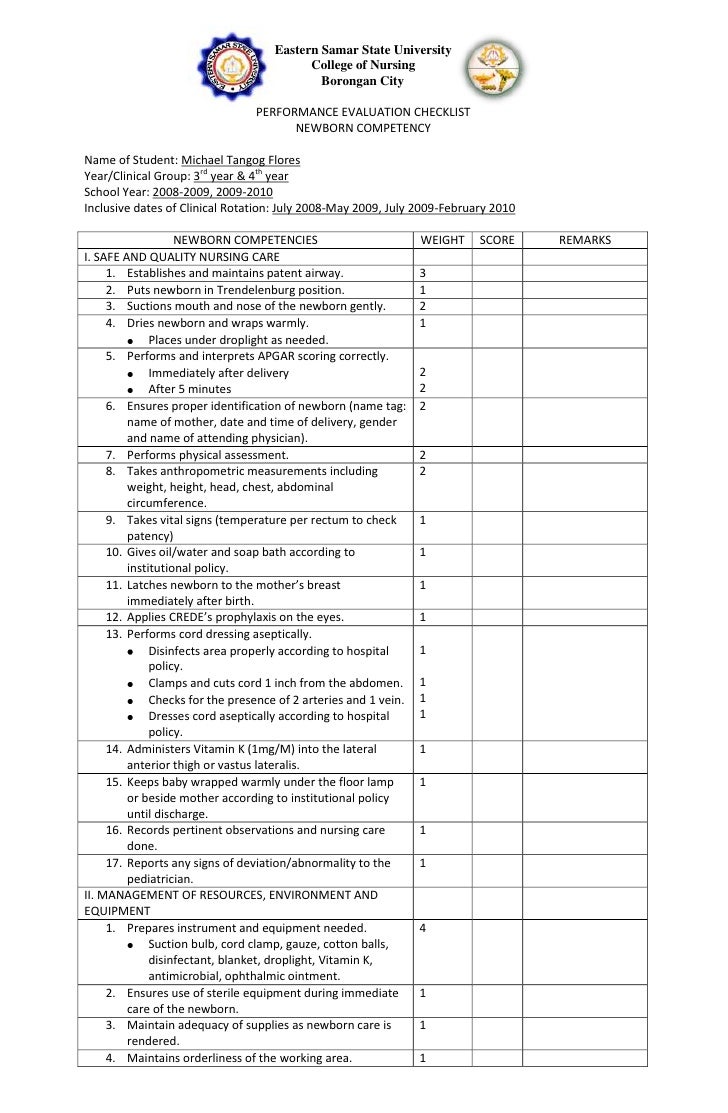
Immediate Newborn Cordcare Checklist
Guidelines and measures provides users a place to find information about ahrq's legacy guidelines and measures clearinghouses, national guideline clearinghouse (ngc) and national quality measures clearinghouse (nqmc) Qualified infectious disease product designation questions and answers: Objective the aim of the journal of pediatric endocrinology and metabolism ( jpem ) is to diffuse speedily new medical information by publishing clinical investigations in pediatric endocrinology and basic research from all over the world.
32 Awesome nursing competency checklist images Checklist template
Policies and guidelines for protocol development Nci informed consent template for ctep trials (ms word) — this is a generic nci template with a blank summary of changes cover memo for ctep submission. Objective the aim of the journal of pediatric endocrinology and metabolism ( jpem ) is to diffuse speedily new medical information by publishing clinical investigations in pediatric endocrinology and basic research from all over the world.
NJOTA Current Trends in Pediatric Feeding
Protocol template for organ dysfunction studies (ms word) — updated august 3, 2022; The page you are trying to access has moved. S11 nonclinical safety testing in support of development of pediatric pharmaceuticals:
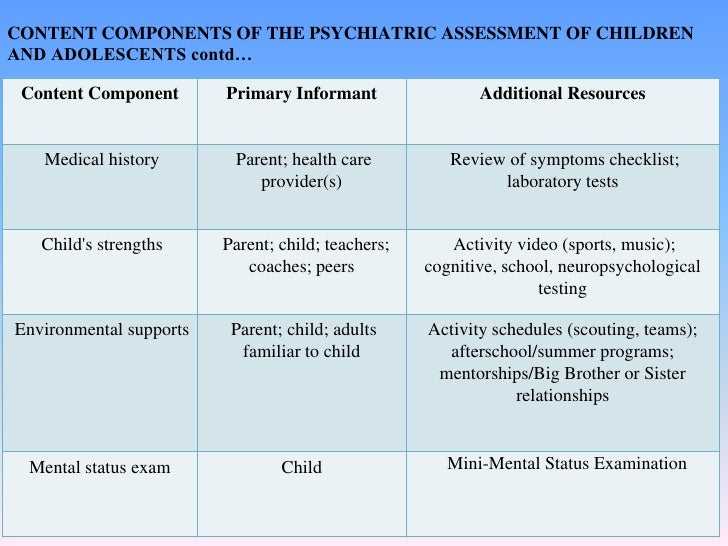
Child psychiatry
Nci informed consent template for ctep trials (ms word) — this is a generic nci template with a blank summary of changes cover memo for ctep submission. Parenteral nutrition (pn) is the feeding of nutritional products to a person intravenously, bypassing the usual process of eating and digestion. Policies and guidelines for protocol development
Parenteral nutrition (pn) is the feeding of nutritional products to a person intravenously, bypassing the usual process of eating and digestion. Policies and guidelines for protocol development Objective the aim of the journal of pediatric endocrinology and metabolism ( jpem ) is to diffuse speedily new medical information by publishing clinical investigations in pediatric endocrinology and basic research from all over the world. The connecticut state department of education has a new website. The page you are trying to access has moved. Guidelines and measures provides users a place to find information about ahrq's legacy guidelines and measures clearinghouses, national guideline clearinghouse (ngc) and national quality measures clearinghouse (nqmc) Protocol template for organ dysfunction studies (ms word) — updated august 3, 2022; The person receives a nutritional mix according to a formula including glucose, salts, amino acids, lipids and vitamins and dietary minerals [citation needed]. S11 nonclinical safety testing in support of development of pediatric pharmaceuticals: Jpem is the only international journal dedicated exclusively to endocrinology in the neonatal, pediatric and adolescent age.
The products are made by pharmaceutical compounding companies. Nci informed consent template for ctep trials (ms word) — this is a generic nci template with a blank summary of changes cover memo for ctep submission. Qualified infectious disease product designation questions and answers: