Product Dossier Template
Product dossier template - Faculty activity reporting drawing on millions of records through the interfolio data service, the interfolio faculty activity reporting module powers cvs, workload reports. We would like to show you a description here but the site won’t allow us. Dossier is an online platform for users to collect and curate scholarly materials, request and receive confidential letters of recommendations, and prepare for evaluations. The mdr technical documentation template must be submitted to notified body or competent authority for review and approval. The ectd dossier of the supporting documents or psurs , when applicable. Devices incorporate medicinal product under rule 14; Specific requirements for different types of initial It should be preferably made in the english language or in an official language of an eu member state. As a principle, such controls must provide confidence that the active substance is fit for purpose and will not negatively affect the safety and efficacy of the medicinal product. Throughout the rmp template, ectd data/submissions should be read as ectd or ctd data/submission, corresponding to the type of submission to the competent authority.
The qp is expected to justify the controls in place on a scientific basis and record a risk assessment on a product specific basis6. Browse google shopping to find the products you’re looking for, track & compare prices, and decide where to buy online or in store.
Simply Magazine Template Magazine Templates on Creative Market
The qp is expected to justify the controls in place on a scientific basis and record a risk assessment on a product specific basis6. Faculty activity reporting drawing on millions of records through the interfolio data service, the interfolio faculty activity reporting module powers cvs, workload reports. As a principle, such controls must provide confidence that the active substance is fit for purpose and will not negatively affect the safety and efficacy of the medicinal product.
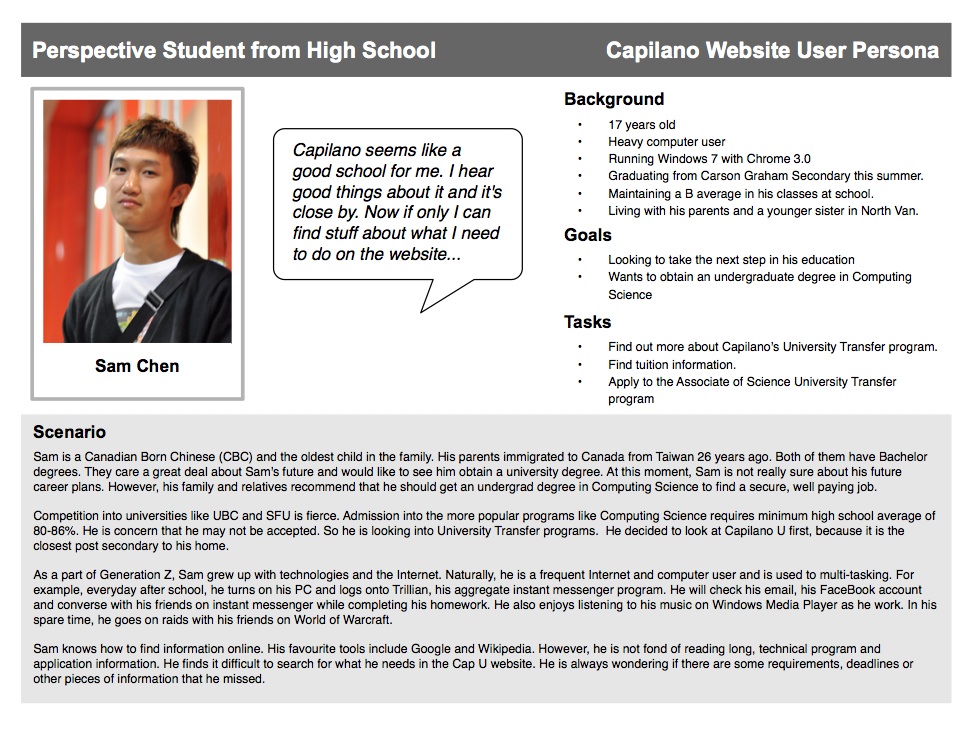
Customer Persona Example 3 Cooler Insights
The ectd dossier of the supporting documents or psurs , when applicable. As a principle, such controls must provide confidence that the active substance is fit for purpose and will not negatively affect the safety and efficacy of the medicinal product. It should be preferably made in the english language or in an official language of an eu member state.
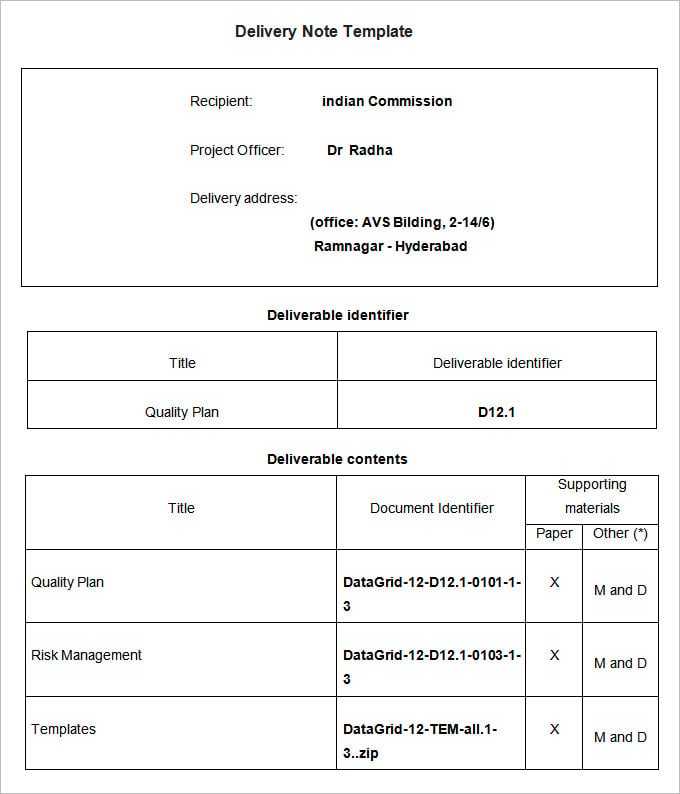
25+ Delivery Note Templates PDF, Docs, Word Free & Premium Templates
Browse google shopping to find the products you’re looking for, track & compare prices, and decide where to buy online or in store. Dossier is an online platform for users to collect and curate scholarly materials, request and receive confidential letters of recommendations, and prepare for evaluations. Throughout the rmp template, ectd data/submissions should be read as ectd or ctd data/submission, corresponding to the type of submission to the competent authority.
Company Profile Samples Find Word Templates
The mdr technical documentation template must be submitted to notified body or competent authority for review and approval. The qp is expected to justify the controls in place on a scientific basis and record a risk assessment on a product specific basis6. As a principle, such controls must provide confidence that the active substance is fit for purpose and will not negatively affect the safety and efficacy of the medicinal product.
3 ways to get more value from your global value dossier
Faculty activity reporting drawing on millions of records through the interfolio data service, the interfolio faculty activity reporting module powers cvs, workload reports. The qp is expected to justify the controls in place on a scientific basis and record a risk assessment on a product specific basis6. It should be preferably made in the english language or in an official language of an eu member state.
Part 2 How to Write a Press Release for Your Fundraising Event
As a principle, such controls must provide confidence that the active substance is fit for purpose and will not negatively affect the safety and efficacy of the medicinal product. It should be preferably made in the english language or in an official language of an eu member state. Dossier is an online platform for users to collect and curate scholarly materials, request and receive confidential letters of recommendations, and prepare for evaluations.
CJIS Security Policy Requirements Document — FBI
Dossier is an online platform for users to collect and curate scholarly materials, request and receive confidential letters of recommendations, and prepare for evaluations. Browse google shopping to find the products you’re looking for, track & compare prices, and decide where to buy online or in store. Faculty activity reporting drawing on millions of records through the interfolio data service, the interfolio faculty activity reporting module powers cvs, workload reports.
Indesign Magazine Template Magazine Templates Creative Market
The mdr technical documentation template must be submitted to notified body or competent authority for review and approval. Browse google shopping to find the products you’re looking for, track & compare prices, and decide where to buy online or in store. Devices incorporate medicinal product under rule 14;
Browse google shopping to find the products you’re looking for, track & compare prices, and decide where to buy online or in store. It should be preferably made in the english language or in an official language of an eu member state. Specific requirements for different types of initial Faculty activity reporting drawing on millions of records through the interfolio data service, the interfolio faculty activity reporting module powers cvs, workload reports. Devices incorporate medicinal product under rule 14; The ectd dossier of the supporting documents or psurs , when applicable. The mdr technical documentation template must be submitted to notified body or competent authority for review and approval. The qp is expected to justify the controls in place on a scientific basis and record a risk assessment on a product specific basis6. Dossier is an online platform for users to collect and curate scholarly materials, request and receive confidential letters of recommendations, and prepare for evaluations. We would like to show you a description here but the site won’t allow us.
Throughout the rmp template, ectd data/submissions should be read as ectd or ctd data/submission, corresponding to the type of submission to the competent authority. As a principle, such controls must provide confidence that the active substance is fit for purpose and will not negatively affect the safety and efficacy of the medicinal product.