Protocol Deviation Management Plan Template
Protocol deviation management plan template - Powerful process validation app to ensure product quality and compliance with fda regulations. Human gene therapy review procedures (ppt) other resources. Use iauditor the best mobile inspection app to generate a comprehensive report and ensure risks are at safe levels. Change management plans must take into consideration, an organization's processes, communication protocol, training method, and impact analysis, success metrics, and more. Nih notice march 22, 2016 Using board management software enables the members to follow the protocol procedures by keeping everything they need in one central location in the cloud. 5 of the best process validation report templates: Haccp audit template can be used to identify the hazards, any ccps, and the critical limit deviation. This template also records the past history of the product, corrective action, and future actions needed. 1) process validation report template and process validation protocol templates for 2) equipment qualification, 3) installation qualification, 4) operational qualification, and 5) performance qualification.
(if you have a separate clinical monitoring and data management plan, please reference it and utilize that. A protocol deviation is generally an unplanned excursion from the protocol that is not implemented or intended as a systematic change. The complete data and safety monitoring report template should be included as an appendix. The nih review process for human gene transfer trials; It helps the meeting run smoothly, with members able to make their points and make informed decisions.
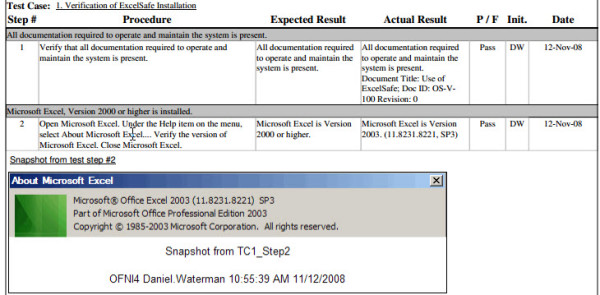
FastVal Installation Qualification Template Ofni Systems
1) process validation report template and process validation protocol templates for 2) equipment qualification, 3) installation qualification, 4) operational qualification, and 5) performance qualification. This template also records the past history of the product, corrective action, and future actions needed. Haccp audit template can be used to identify the hazards, any ccps, and the critical limit deviation.
The nih review process for human gene transfer trials; 1) process validation report template and process validation protocol templates for 2) equipment qualification, 3) installation qualification, 4) operational qualification, and 5) performance qualification. Using board management software enables the members to follow the protocol procedures by keeping everything they need in one central location in the cloud. A protocol deviation is generally an unplanned excursion from the protocol that is not implemented or intended as a systematic change. Human gene therapy review procedures (ppt) other resources. Use iauditor the best mobile inspection app to generate a comprehensive report and ensure risks are at safe levels. It helps the meeting run smoothly, with members able to make their points and make informed decisions. 5 of the best process validation report templates: This template also records the past history of the product, corrective action, and future actions needed. Powerful process validation app to ensure product quality and compliance with fda regulations.
Nih notice march 22, 2016 Change management plans must take into consideration, an organization's processes, communication protocol, training method, and impact analysis, success metrics, and more. (if you have a separate clinical monitoring and data management plan, please reference it and utilize that. Haccp audit template can be used to identify the hazards, any ccps, and the critical limit deviation. The complete data and safety monitoring report template should be included as an appendix.