Quality Manual Template
Quality manual template - Quality assurance for software testing exists to ensure that the product is built correctly without too many iterations. It is the basis for formally testing any software / product in a project. Note that testing should not follow development but should support it. Quality management systems — requirements. For the introduction of the iso 17025 standard, you need: Assemble and deliver your manual. A document describing the scope, approach, resources and schedule of intended test activities.it identifies amongst others test items, the features to be tested, the testing tasks, who will do. Coordinator is the individual responsible for managing qm activities at the clinical site and is named in the clinical quality management plan. The qm coordinator is also likely to be the author of the qm summary report. User our qa manual iso 9001:2015 template for your quality management system.
Don’t spend hundreds of hours developing your own from scratch. There was a need for more pragmatic, service. The process of adding images to a template, document, or another source involves formatting frustrations, image sizing issues, and other similar, unforeseen. Dra information and candidate list We provide iso 17025 implementation packages which help in writing the required documentation and assist with implementation of the accreditation requirements.
13+ Sample Quality Manuals Sample Templates
Quality assurance for software testing exists to ensure that the product is built correctly without too many iterations. Assemble and deliver your manual. The process of adding images to a template, document, or another source involves formatting frustrations, image sizing issues, and other similar, unforeseen.
Construction Superintendent Resume
Assemble and deliver your manual. Coordinator is the individual responsible for managing qm activities at the clinical site and is named in the clinical quality management plan. We provide iso 17025 implementation packages which help in writing the required documentation and assist with implementation of the accreditation requirements.
ISO 90012015 Template Documentation KELBIX
Quality assurance for software testing exists to ensure that the product is built correctly without too many iterations. There was a need for more pragmatic, service. Coordinator is the individual responsible for managing qm activities at the clinical site and is named in the clinical quality management plan.
Free Creative Company Profile Template
Quality management systems — requirements. This human resource manual template includes sample content and guidelines on how you can individually adjust this document to suit the needs of your business. Don’t spend hundreds of hours developing your own from scratch.
Wedding Invitation Format Free Word Templates
It is the basis for formally testing any software / product in a project. A test plan is a document describing software testing scope and activities. Coordinator is the individual responsible for managing qm activities at the clinical site and is named in the clinical quality management plan.
Corporate Governance Guidelines Template SlideModel
Quality assurance for software testing exists to ensure that the product is built correctly without too many iterations. A prerequisite for a laboratory to become accredited is to have a documented quality system. We provide iso 17025 implementation packages which help in writing the required documentation and assist with implementation of the accreditation requirements.
Restaurant Training Manual Templates
Quality assurance for software testing exists to ensure that the product is built correctly without too many iterations. This human resource manual template includes sample content and guidelines on how you can individually adjust this document to suit the needs of your business. Coordinator is the individual responsible for managing qm activities at the clinical site and is named in the clinical quality management plan.
monthly Grocery List Free Word Templates
Note that testing should not follow development but should support it. A prerequisite for a laboratory to become accredited is to have a documented quality system. It describes top level standard operating procedures, processes and specifications.
It is the basis for formally testing any software / product in a project. Don’t spend hundreds of hours developing your own from scratch. We provide iso 17025 implementation packages which help in writing the required documentation and assist with implementation of the accreditation requirements. 1 the example manual also demonstrates that a single manual can be used to show conformance or compliance. Note that testing should not follow development but should support it. Edit all of your images to prepare them for inclusion in your training manual. It describes top level standard operating procedures, processes and specifications. User our qa manual iso 9001:2015 template for your quality management system. The process of adding images to a template, document, or another source involves formatting frustrations, image sizing issues, and other similar, unforeseen. Dra information and candidate list
Quality assurance for software testing exists to ensure that the product is built correctly without too many iterations. The story of {business name} starts in 2008 when janet howie and lucy smith saw a gap in the sme market for quality accounting services. Quality management systems — requirements. Your customer will use it as a reference guide. Now is when creating a training manual usually becomes tedious. A test plan is a document describing software testing scope and activities. There was a need for more pragmatic, service. This human resource manual template includes sample content and guidelines on how you can individually adjust this document to suit the needs of your business. Use this template to quickly. A worthwhile qa process clearly defines requirements, gives testers a thorough understanding of the features, and gives them a blueprint for how to progress.
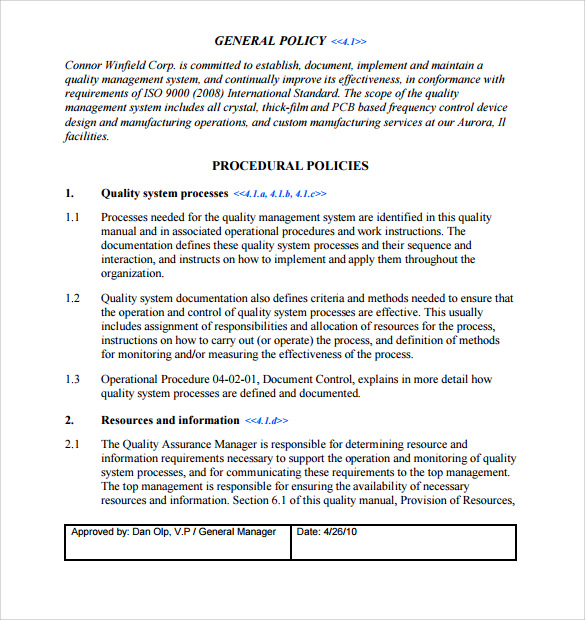
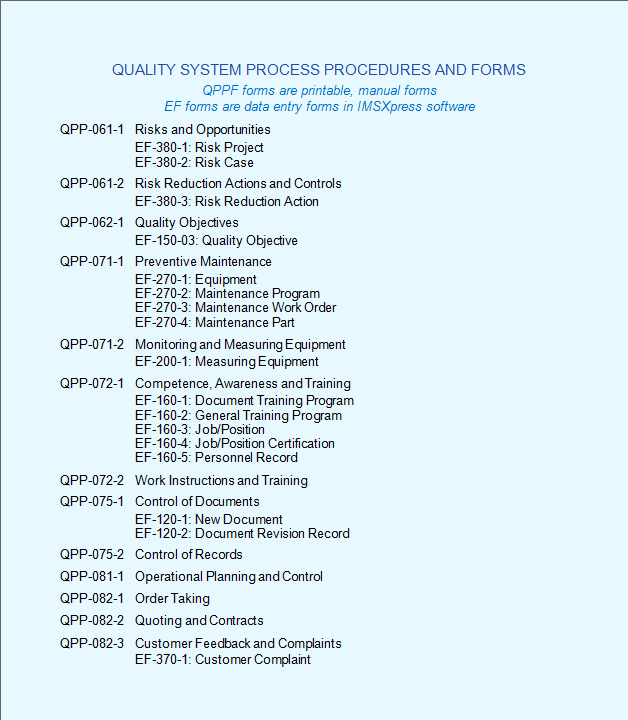
A prerequisite for a laboratory to become accredited is to have a documented quality system. Your quality manual is the top level document that specifies your quality management system. The qm coordinator is also likely to be the author of the qm summary report. Assemble and deliver your manual. For the introduction of the iso 17025 standard, you need: Coordinator is the individual responsible for managing qm activities at the clinical site and is named in the clinical quality management plan. A document describing the scope, approach, resources and schedule of intended test activities.it identifies amongst others test items, the features to be tested, the testing tasks, who will do.