Substance Template
Substance template - The format of the qp declaration template is in five parts (parts a to e). Active substance manufactured in accordance with the above active substance master file will no longer be supplied after [supply agreement termination date], replacement of the active substance master file by certificate of suitability, [cep no]. The attached qp declaration template provides a suitable means for documenting confirmation that the active substance manufacture complies with gmp requirements. A copy of the certificate of suitability is attached to this letter Concerned active substance manufacturing sites the name of the active substance should be stated.
Examples of different Hazardous substances, New Zealand (NZ). Stock
Concerned active substance manufacturing sites the name of the active substance should be stated. Active substance manufactured in accordance with the above active substance master file will no longer be supplied after [supply agreement termination date], replacement of the active substance master file by certificate of suitability, [cep no]. The format of the qp declaration template is in five parts (parts a to e).
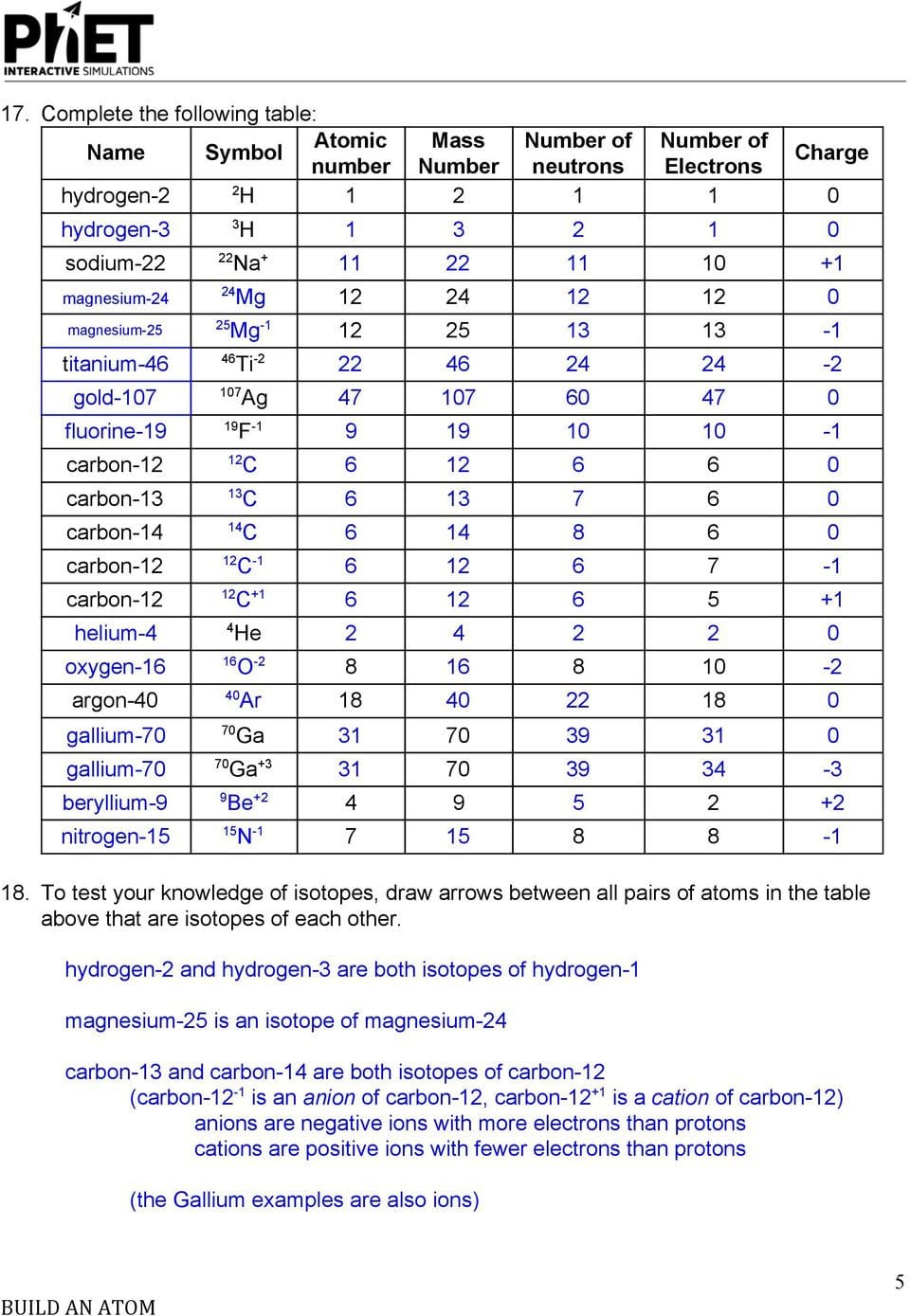
Answer Key Build An Atom Part I Atom Screen Build An Atom —
A copy of the certificate of suitability is attached to this letter Active substance manufactured in accordance with the above active substance master file will no longer be supplied after [supply agreement termination date], replacement of the active substance master file by certificate of suitability, [cep no]. The attached qp declaration template provides a suitable means for documenting confirmation that the active substance manufacture complies with gmp requirements.
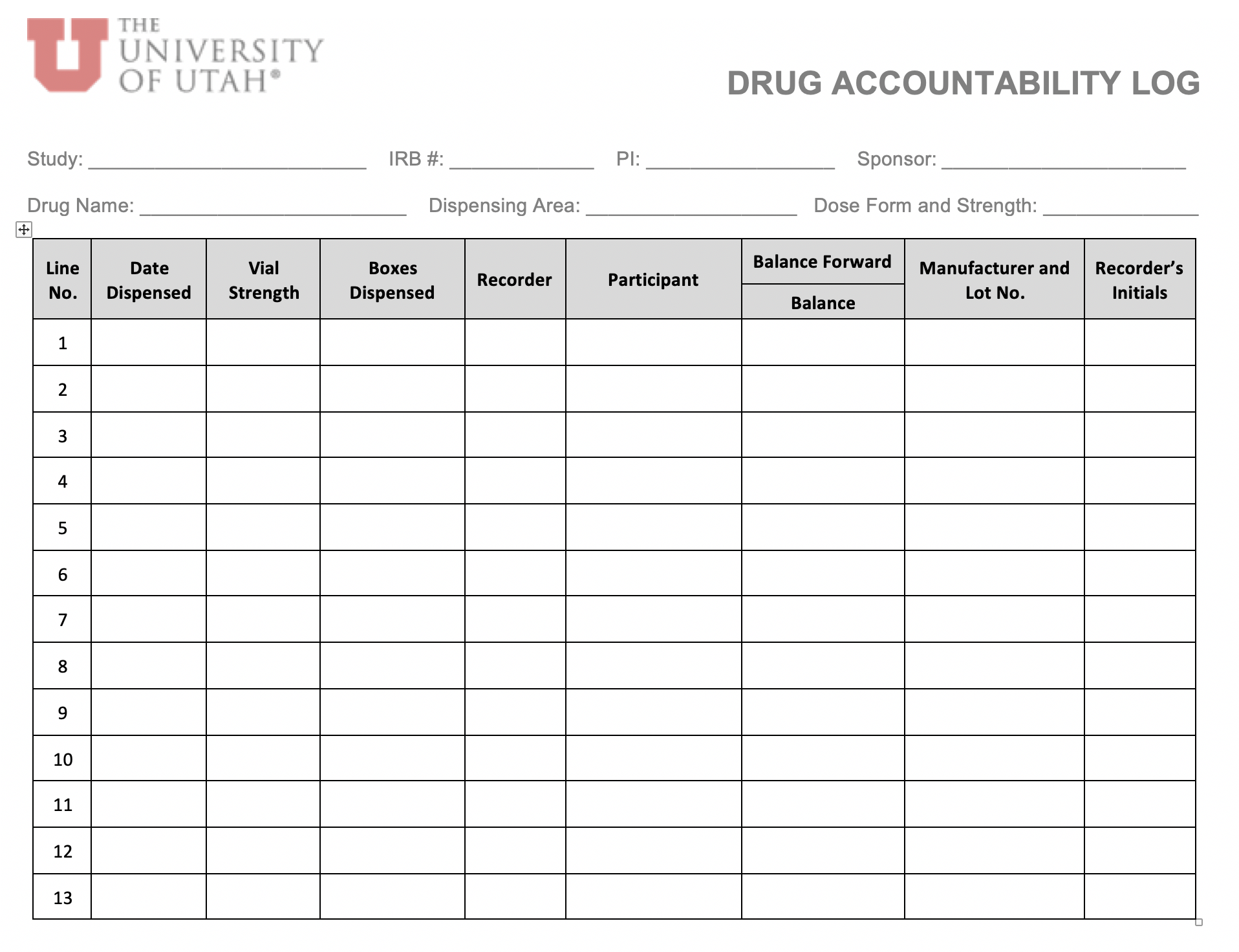
Tool Kit Research Quality and Compliance The University of Utah
The attached qp declaration template provides a suitable means for documenting confirmation that the active substance manufacture complies with gmp requirements. Active substance manufactured in accordance with the above active substance master file will no longer be supplied after [supply agreement termination date], replacement of the active substance master file by certificate of suitability, [cep no]. The format of the qp declaration template is in five parts (parts a to e).
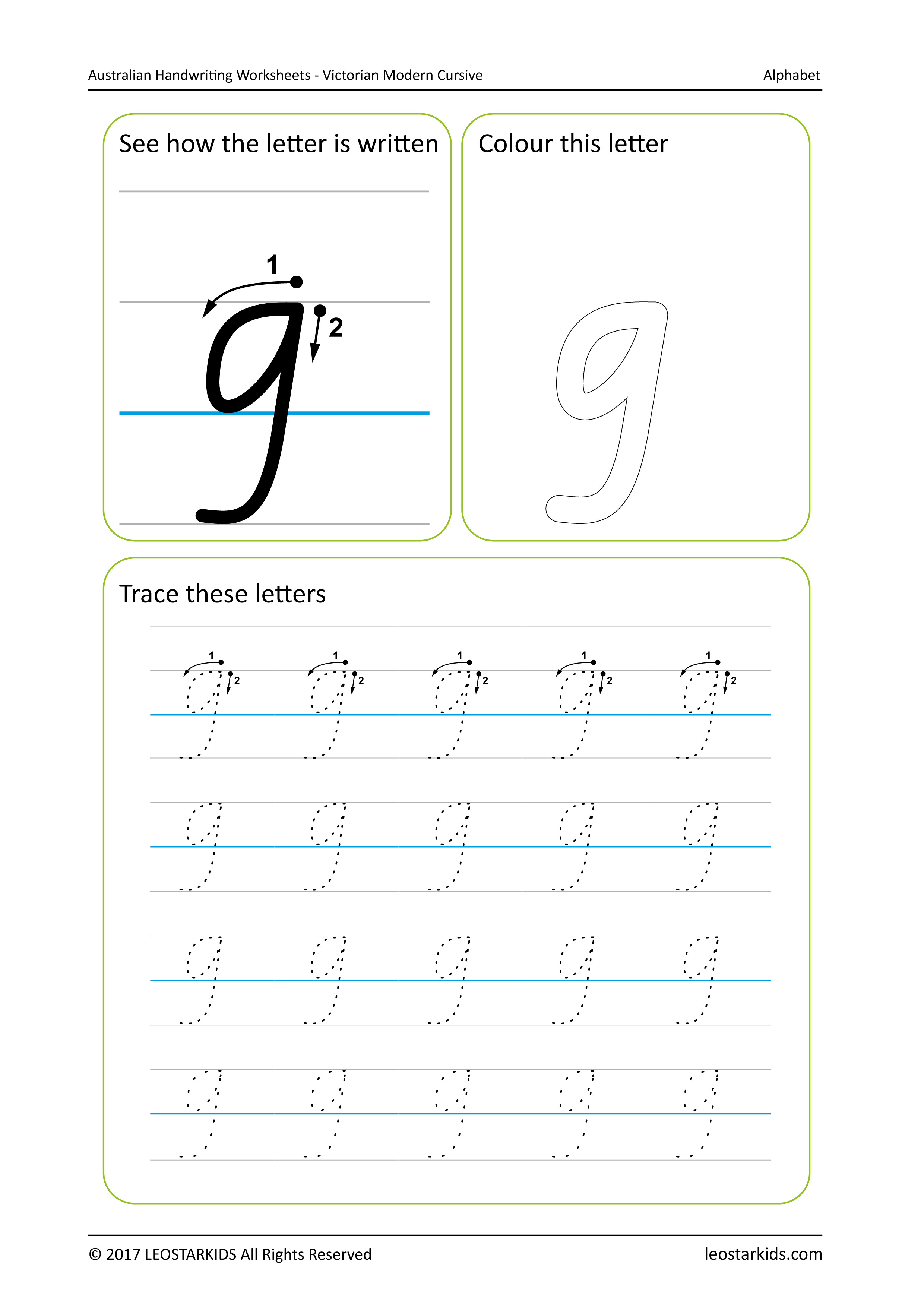
Australian Handwriting Worksheets Victorian Modern Cursive —
Concerned active substance manufacturing sites the name of the active substance should be stated. The attached qp declaration template provides a suitable means for documenting confirmation that the active substance manufacture complies with gmp requirements. Active substance manufactured in accordance with the above active substance master file will no longer be supplied after [supply agreement termination date], replacement of the active substance master file by certificate of suitability, [cep no].
Clinical Counselor Resume Samples QwikResume
The format of the qp declaration template is in five parts (parts a to e). A copy of the certificate of suitability is attached to this letter The attached qp declaration template provides a suitable means for documenting confirmation that the active substance manufacture complies with gmp requirements.
Substance Abuse Counselor Intern Resume Samples QwikResume
The format of the qp declaration template is in five parts (parts a to e). Concerned active substance manufacturing sites the name of the active substance should be stated. A copy of the certificate of suitability is attached to this letter
Treatment Planning Mastering Competencies 2nd edition YouTube
The attached qp declaration template provides a suitable means for documenting confirmation that the active substance manufacture complies with gmp requirements. The format of the qp declaration template is in five parts (parts a to e). Concerned active substance manufacturing sites the name of the active substance should be stated.
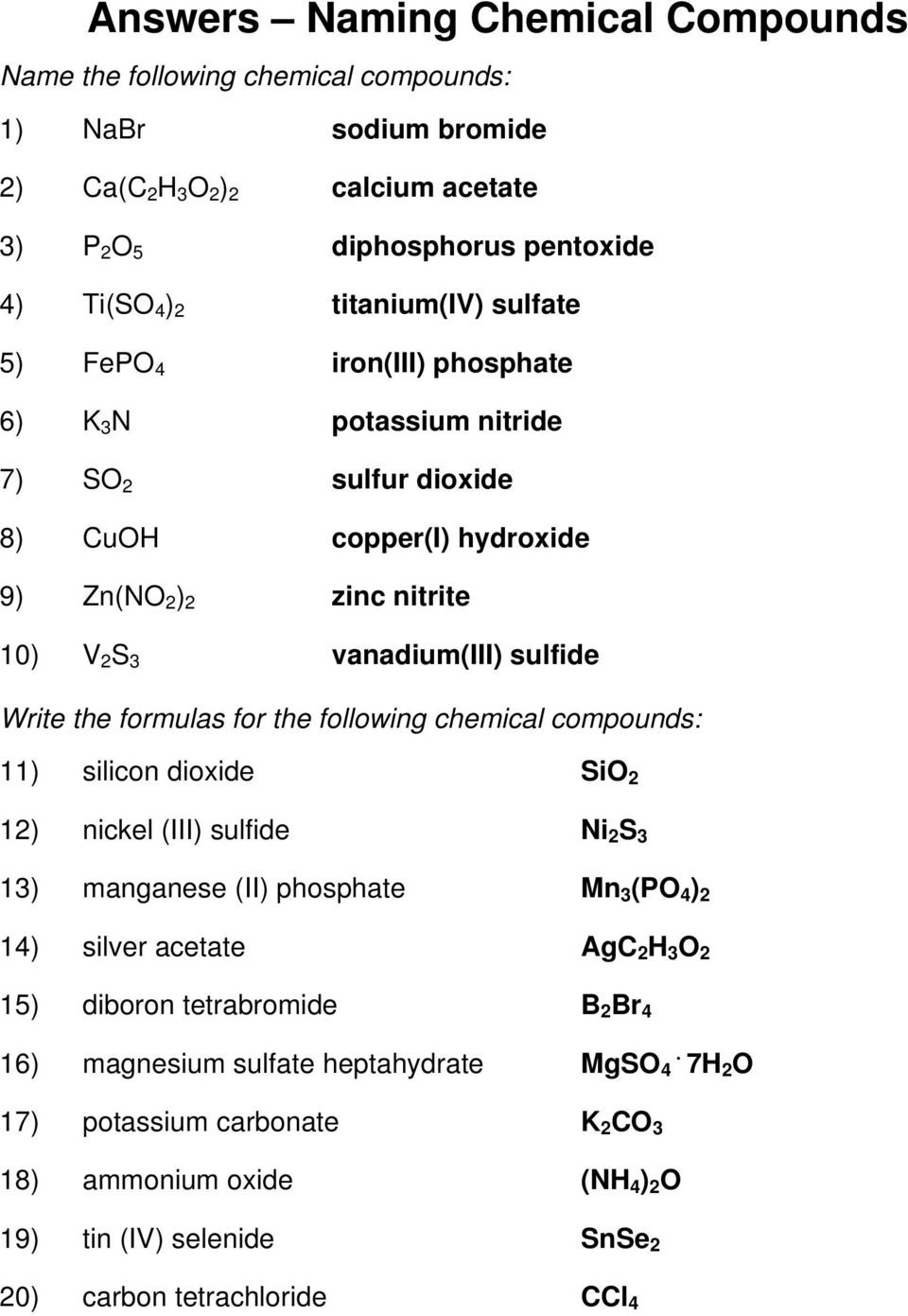
Naming Ionic Compounds Answer Key Pdf —
A copy of the certificate of suitability is attached to this letter Active substance manufactured in accordance with the above active substance master file will no longer be supplied after [supply agreement termination date], replacement of the active substance master file by certificate of suitability, [cep no]. The attached qp declaration template provides a suitable means for documenting confirmation that the active substance manufacture complies with gmp requirements.
A copy of the certificate of suitability is attached to this letter The format of the qp declaration template is in five parts (parts a to e). Active substance manufactured in accordance with the above active substance master file will no longer be supplied after [supply agreement termination date], replacement of the active substance master file by certificate of suitability, [cep no]. Concerned active substance manufacturing sites the name of the active substance should be stated. The attached qp declaration template provides a suitable means for documenting confirmation that the active substance manufacture complies with gmp requirements.